With the enhancement of public health awareness and the improvement of drug safety standards, controlling pesticide residues in traditional Chinese medicinal materials has become particularly crucial. The 2025 edition of the "Chinese Pharmacopoeia" draft for pesticide residue detection methods has been comprehensively revised and updated to ensure the safety and effectiveness of medicinal materials. This article outlines the important revisions and provides corresponding solutions.
Draft explanation for the 2341 Pesticide Residue Quantification Method
Proposes to delete the original Methods 1, 2, and 3.
Improve the method for determining residues of banned pesticides. Proposes systematic research, refinement, and integration of the preparation and purification methods for test solutions in the current standards, and has completed cross-verification.
Collect representative market samples and propose new detection methods for specific pesticides and medicinal material matrices, and complete cross-verification.
Propose new detection methods for dithiocarbamate pesticides in certain traditional Chinese medicinal varieties (lily, Panax notoginseng) and complete cross-verification.
Note: The above content is excerpted from the original draft document.
Draft explanation for the 0212 General Principles of Identification for Medicinal Materials and Decoction Pieces
Improve the index for banned pesticides in traditional Chinese medications. Summarize and organize the directory of banned pesticides in traditional Chinese medications released by the Ministry of Agriculture and Rural Affairs from 2017 to present, and compile their toxicity, detection methods, and limit standards. Update the types and limitations of banned pesticides to be added to the Pharmacopoeia.
Sort and classify the 10 types of traditional Chinese medications involved in GB 2763-2021 and GB 2763.1-2022, formulate the conversion principles for the maximum residue limits of pesticides, and based on the daily allowable intake (ADI) set by the Joint FAO/WHO Meeting on Pesticide Residues (JMPR), conduct a health risk assessment. Propose converting the maximum residue limits of pesticides for the 10 types of traditional Chinese medications involved in GB 2763-2021 into the standards for the "Chinese Pharmacopoeia".
Considering the current situation of paclobutrazol residues in Radix Ophiopogonis, the impact of paclobutrazol use on the quality of Radix Ophiopogonis, and the extraction transfer rate results of paclobutrazol residues in Radix Ophiopogonis, a standard for the maximum limit of paclobutrazol in Radix Ophiopogonis has been established.
Note: The above content is excerpted from the original draft document.
Contents of the 2341 Pesticide Residue Quantification Method Draft Standard in the Pharmacopoeia
Delete the original first, second, and third methods, as well as the original fifth method for determining multiple pesticide residues in herbal medicines and decoction pieces (plant-based).
Change to the first method and revise it, increasing the number of banned pesticide residues from 33 to 47, and adding new second and third methods.
| 2020 Edition of Pharmacopoeia 2341 General Principle | 2025 Edition of Pharmacopoeia 2341 General Principle Draft |
First Method: Determination of Organochlorine Pesticide Residues (Chromatography Method) | First Method (Formerly the Fifth Method): Determination of Multiple Banned Pesticide Residues in Herbal Medicines and Decoction Pieces (Plant-based) |
Second Method: Determination of Organophosphorus Pesticide Residues (Chromatography Method) | Second Method: Determination of Multiple Pesticide Residues in Related Herbal Medicines and Decoction Pieces Method |
Third Method: Determination of Pyrethroid Pesticide Residues (Chromatography Method) | Third Method: Determination of Dithiocarbamate Pesticide Residues in Herbal Medicines and Decoction Pieces |
Fourth Method: Determination of Multiple Pesticide Residues (Mass Spectrometry Method) | Fourth Method: Determination of Multiple Pesticide Residues (Mass Spectrometry Method) |
| (Plant-based) Original Fifth Method: Determination of Multiple Pesticide Residues in Herbal Medicines and Decoction Pieces | / |
Summary of Revisions to the General Principles for Banned Pesticides in Herbal Medicines and Decoction Pieces (Plant-based)
Solution Preparation

Detected Compounds and Reporting Limits
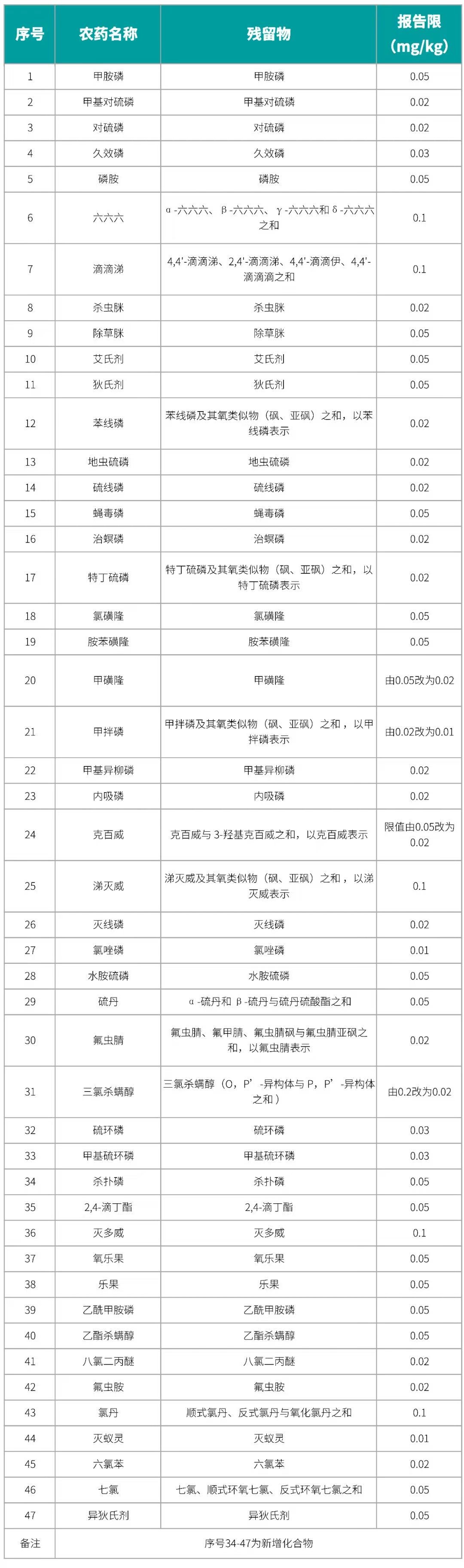
Detection Conditions and Quantification Method

Summary of Multiple Pesticide Residues in Related Herbal Medicines and Decoction Pieces
Solution Preparation

Detected Compounds and Reporting Limits

Detection Conditions and Quantification Method: Same as the First Method
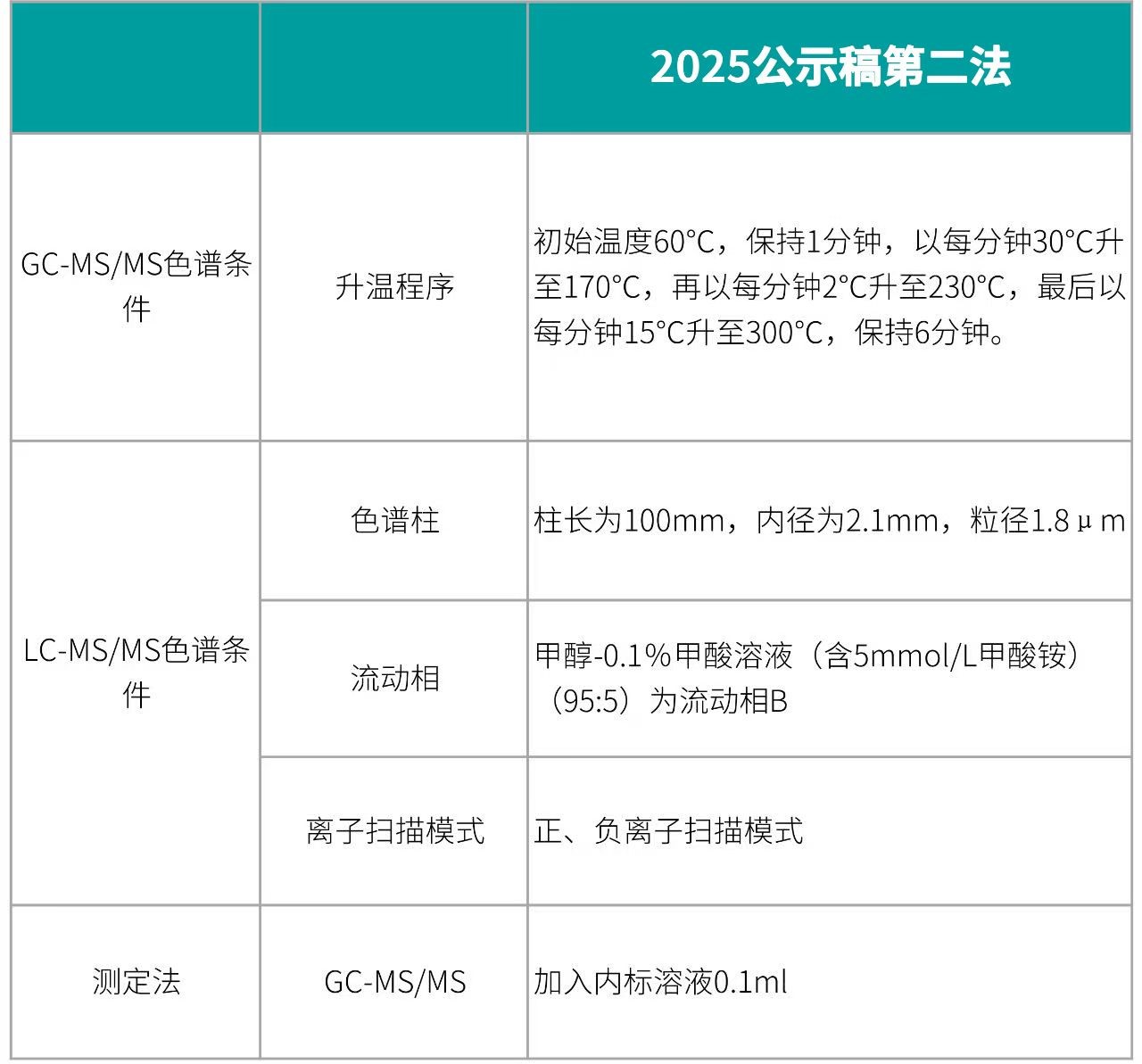
Raykol's Applicable Equipment:
solid phase extraction equipment
